Exam 17: Allylic and Benzylic Reactivity
These compounds have very different acidities. State which is the most acidic, which is the least acidic, which is in the middle, and why.
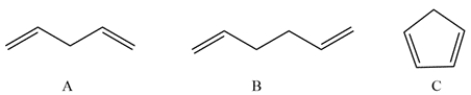
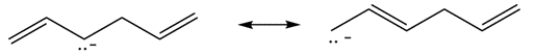
Each of the compounds contains an allylic proton. The allylic protons in compound A are double allylic since it is next to two double bonds. The resulting carbanion can be delocalized to give a stable carbanion.
 Compound B contains two equivalent allylic protons. There are only two resonance structures possible.
Compound B contains two equivalent allylic protons. There are only two resonance structures possible.
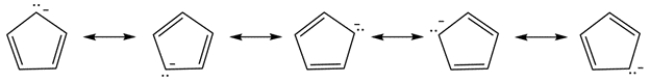
 Compound C also contains a doubly allylic proton. In addition, the resulting carbanion results in an aromatic ring, which is very stable. The charge can be delocalized to each carbon in the ring.
Compound C also contains a doubly allylic proton. In addition, the resulting carbanion results in an aromatic ring, which is very stable. The charge can be delocalized to each carbon in the ring.
 The most acidic compounds will have the most stable conjugate base. Thus, compound C is the most acidic, followed by compound A, then compound B.
The most acidic compounds will have the most stable conjugate base. Thus, compound C is the most acidic, followed by compound A, then compound B.
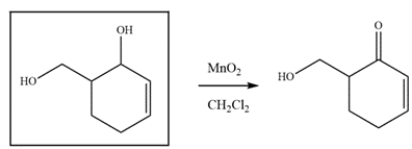
The compound was synthesized from MnO2 oxidation. Deduce the structure of the starting material.
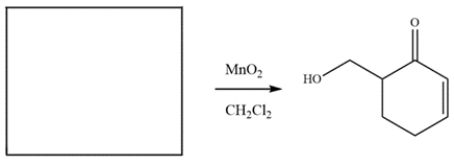
Manganese dioxide oxidizes allylic alcohols to aldehyde or ketones, so the ketone must have been an alcohol.
A bottle labeled 3-iodo-1-pentene (structure below) was stored in the back of the laboratory refrigerator. Several weeks later, a chemist analyzed the compound and found that it was contaminated with a different molecule with the same molecular formula. Determine the structure of the isomer and draw a curved-arrow mechanism to explain how it was formed.
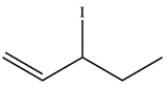
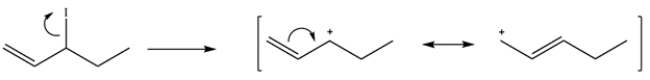
3-Iodo-1-pentene contains an allylic iodide. Loss of the iodide leaving group leaves a secondary carbocation, which has a resonance structure with a primary carbocation.
 The iodide ion can attack resonance structure 1 to regenerate 3-iodo-1-pentene, or it can attack resonance structure 2 to form 1-iodo-2-pentene.
The iodide ion can attack resonance structure 1 to regenerate 3-iodo-1-pentene, or it can attack resonance structure 2 to form 1-iodo-2-pentene.
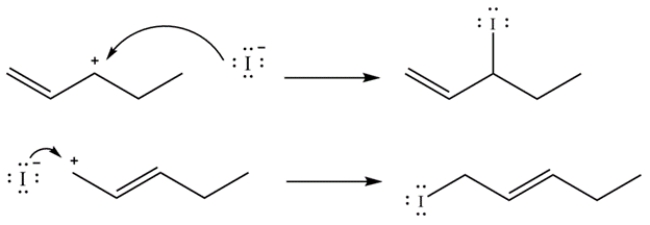 Note, the 1-iodo-2-pentene is the thermodynamic product since it is a disubstituted alkene, as compared to 3-iodo-1-pentene, which is a monosubstituted alkene.
Note, the 1-iodo-2-pentene is the thermodynamic product since it is a disubstituted alkene, as compared to 3-iodo-1-pentene, which is a monosubstituted alkene.
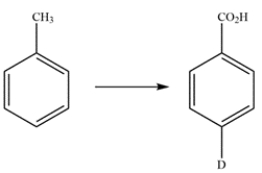
Propose a multistep synthesis for the compound from toluene. You may assume that ortho and para isomers can be separated as necessary. Reaction mechanisms are not necessary.
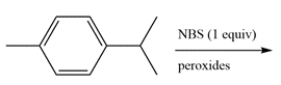
Determine the major organic product for the reaction. The product should have a molecular formula of C10H13Br.
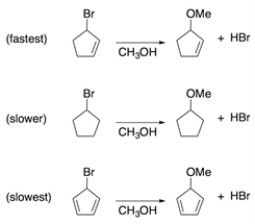
Rationalize these two observations through analysis of intermediates.
1. Solvolysis of bromocyclopentene in methanol is much faster than is solvolysis of bromocyclopentane.
2. Solvolysis of bromocyclopentadiene is slower than solvolysis of bromocyclopentane.
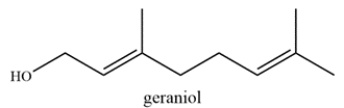
The structure of geraniol is shown. Label the allylic carbons with an asterisk (*).
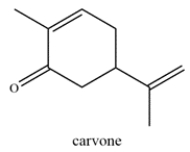
Carvone is a terpenoid that is found in essential oils and used for aromatherapy. Highlight the isoprene units in carvone and classify the terpene based on the number of carbons (ex. monoterpene, etc.)
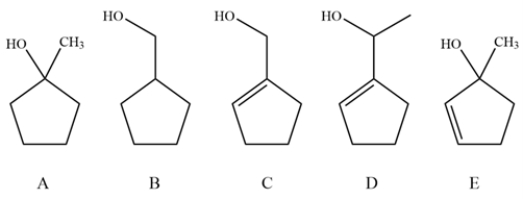
Consider these alcohols and explain whether oxidation with pyridinium chlorochromate (PCC) and MnO2 would give the same product, different products or no reaction.
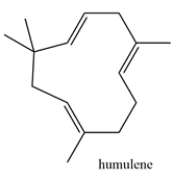
Humulene is a terpene that is responsible for the hops aroma in beer. Highlight the isoprene units in humulene and classify the terpene based on the number of carbons (ex. monoterpene, etc.)
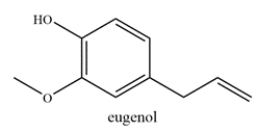
Consider the structure of eugenol, which is commonly found in essential oils and is used in perfumes.
 Which statement is true?
Which statement is true?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)