Exam 8: Energy and Enzymes: an Introduction to Metabolism
Exam 1: Biology and the Tree of Life37 Questions
Exam 2: Water and Carbon: the Chemical Basis of Life59 Questions
Exam 3: Protein Structure and Function59 Questions
Exam 4: Nucleic Acids and the Rna World43 Questions
Exam 5: An Introduction to Carbohydrates44 Questions
Exam 53: Ecosystems and Global Ecology57 Questions
Exam 6: Lipids, Membranes, and the First Cells59 Questions
Exam 7: Inside the Cell60 Questions
Exam 8: Energy and Enzymes: an Introduction to Metabolism60 Questions
Exam 9: Cellular Respiration and Fermentation61 Questions
Exam 10: Photosynthesis58 Questions
Exam 11: Cellcell Interactions52 Questions
Exam 12: The Cell Cycle59 Questions
Exam 13: Meiosis63 Questions
Exam 14: Mendel and the Gene60 Questions
Exam 15: Dna and the Gene: Synthesis and Repair51 Questions
Exam 16: How Genes Work48 Questions
Exam 17: Transcription, Rna Processing, and Translation58 Questions
Exam 18: Control of Gene Expression in Bacteria29 Questions
Exam 19: Control of Gene Expression in Eukaryotes56 Questions
Exam 20: The Molecular Revolution: Biotechnology and Beyond70 Questions
Exam 21: Genes, Development, and Evolution38 Questions
Exam 22: Evolution by Natural Selection38 Questions
Exam 23: Evolutionary Processes37 Questions
Exam 24: Speciation56 Questions
Exam 25: Phylogenies and the History of Life63 Questions
Exam 26: Bacteria and Archaea38 Questions
Exam 27: Protists37 Questions
Exam 28: Green Algae and Land Plants59 Questions
Exam 29: Fungi47 Questions
Exam 30: An Introduction to Animals48 Questions
Exam 31: Protostome Animals54 Questions
Exam 32: Deuterostome Animals60 Questions
Exam 33: Viruses44 Questions
Exam 34: Plant Form and Function46 Questions
Exam 35: Water and Sugar Transport in Plants47 Questions
Exam 36: Plant Nutrition54 Questions
Exam 37: Plant Sensory Systems, Signals, and Responses48 Questions
Exam 38: Plant Reproduction and Development51 Questions
Exam 39: Animal Form and Function53 Questions
Exam 40: Water and Electrolyte Balance in Animals60 Questions
Exam 41: Animal Nutrition94 Questions
Exam 42: Gas Exchange and Circulation93 Questions
Exam 43: Animal Nervous Systems100 Questions
Exam 44: Animal Sensory Systems50 Questions
Exam 45: Animal Movement40 Questions
Exam 46: Chemical Signals in Animals59 Questions
Exam 47: Animal Reproduction and Development104 Questions
Exam 48: The Immune System in Animals77 Questions
Exam 49: An Introduction to Ecology40 Questions
Exam 50: Behavioral Ecology40 Questions
Exam 51: Population Ecology57 Questions
Exam 52: Community Ecology55 Questions
Exam 54: Biodiversity and Conservation Biology43 Questions
Select questions type
 -You have discovered an enzyme that can catalyze two different chemical reactions. Which of the following is most likely to be correct?
-You have discovered an enzyme that can catalyze two different chemical reactions. Which of the following is most likely to be correct?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
Use the following information to answer the question(s) below.
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
-Some bacteria are metabolically active in hot springs because ________.
Free
(Multiple Choice)
4.7/5  (31)
(31)
Correct Answer:
C
A chemical reaction that has a positive ΔG is best described as ________.
(Multiple Choice)
4.8/5  (45)
(45)
 -Consider the HIV enzyme called protease. The amino acid residues at the active site are highly hydrophobic. In designing a drug that would bind to the active site and jam it, researchers should use a molecule that is ________.
-Consider the HIV enzyme called protease. The amino acid residues at the active site are highly hydrophobic. In designing a drug that would bind to the active site and jam it, researchers should use a molecule that is ________.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is an example of potential rather than kinetic energy?
(Multiple Choice)
4.9/5  (31)
(31)
During a laboratory experiment, you discover that an enzyme-catalyzed reaction has a ∆G of -20 kcal/mol. If you double the amount of enzyme in the reaction, what will be the ∆G for the new reaction?
(Multiple Choice)
4.8/5  (40)
(40)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure. 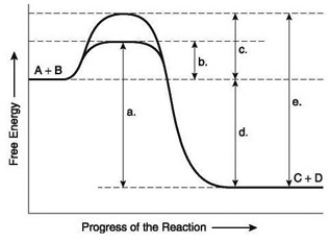 -Which of the following represents the activation energy needed for the enzyme-catalyzed reverse reaction, C + D → A + B, in the figure?
-Which of the following represents the activation energy needed for the enzyme-catalyzed reverse reaction, C + D → A + B, in the figure?
(Multiple Choice)
4.9/5  (39)
(39)
Use the following information to answer the question(s) below.
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
-With respect to the enzyme that converts X to Y, substance A functions as ________.
(Multiple Choice)
4.8/5  (40)
(40)
To attach a particular amino acid to the tRNA molecule that will transport it, an enzyme, an aminoacyl-tRNA synthetase, is required, along with ATP. Initially, the enzyme has an active site for ATP and another for the amino acid, but it is not able to attach the tRNA. What must occur for the final attachment to occur?
(Multiple Choice)
4.7/5  (22)
(22)
According to the induced fit hypothesis of enzyme catalysis, ________.
(Multiple Choice)
4.8/5  (35)
(35)
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following?
(Multiple Choice)
4.9/5  (31)
(31)
The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is most precisely described as ________.
(Multiple Choice)
5.0/5  (38)
(38)
You collect data on the effect of pH on the function of the enzyme catalase in human cells. Which of the following graphs would you expect?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following statements is a logical consequence of the second law of thermodynamics?
(Multiple Choice)
4.8/5  (30)
(30)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -HIV is the virus that causes AIDS. In the mid-1990s, researchers discovered an enzyme in HIV called protease. Once the enzyme's structure was known, researchers began looking for drugs that would fit into the active site and block it. If this strategy for stopping HIV infections were successful, it would be an example of what phenomenon?
-HIV is the virus that causes AIDS. In the mid-1990s, researchers discovered an enzyme in HIV called protease. Once the enzyme's structure was known, researchers began looking for drugs that would fit into the active site and block it. If this strategy for stopping HIV infections were successful, it would be an example of what phenomenon?
(Multiple Choice)
4.8/5  (32)
(32)
Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as ________ is to ________.
(Multiple Choice)
4.9/5  (33)
(33)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -Which of the following terms best describes the forward reaction in the figure?
-Which of the following terms best describes the forward reaction in the figure?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 60
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)