Exam 8: Energy and Enzymes: an Introduction to Metabolism
Exam 1: Biology and the Tree of Life37 Questions
Exam 2: Water and Carbon: the Chemical Basis of Life59 Questions
Exam 3: Protein Structure and Function59 Questions
Exam 4: Nucleic Acids and the Rna World43 Questions
Exam 5: An Introduction to Carbohydrates44 Questions
Exam 53: Ecosystems and Global Ecology57 Questions
Exam 6: Lipids, Membranes, and the First Cells59 Questions
Exam 7: Inside the Cell60 Questions
Exam 8: Energy and Enzymes: an Introduction to Metabolism60 Questions
Exam 9: Cellular Respiration and Fermentation61 Questions
Exam 10: Photosynthesis58 Questions
Exam 11: Cellcell Interactions52 Questions
Exam 12: The Cell Cycle59 Questions
Exam 13: Meiosis63 Questions
Exam 14: Mendel and the Gene60 Questions
Exam 15: Dna and the Gene: Synthesis and Repair51 Questions
Exam 16: How Genes Work48 Questions
Exam 17: Transcription, Rna Processing, and Translation58 Questions
Exam 18: Control of Gene Expression in Bacteria29 Questions
Exam 19: Control of Gene Expression in Eukaryotes56 Questions
Exam 20: The Molecular Revolution: Biotechnology and Beyond70 Questions
Exam 21: Genes, Development, and Evolution38 Questions
Exam 22: Evolution by Natural Selection38 Questions
Exam 23: Evolutionary Processes37 Questions
Exam 24: Speciation56 Questions
Exam 25: Phylogenies and the History of Life63 Questions
Exam 26: Bacteria and Archaea38 Questions
Exam 27: Protists37 Questions
Exam 28: Green Algae and Land Plants59 Questions
Exam 29: Fungi47 Questions
Exam 30: An Introduction to Animals48 Questions
Exam 31: Protostome Animals54 Questions
Exam 32: Deuterostome Animals60 Questions
Exam 33: Viruses44 Questions
Exam 34: Plant Form and Function46 Questions
Exam 35: Water and Sugar Transport in Plants47 Questions
Exam 36: Plant Nutrition54 Questions
Exam 37: Plant Sensory Systems, Signals, and Responses48 Questions
Exam 38: Plant Reproduction and Development51 Questions
Exam 39: Animal Form and Function53 Questions
Exam 40: Water and Electrolyte Balance in Animals60 Questions
Exam 41: Animal Nutrition94 Questions
Exam 42: Gas Exchange and Circulation93 Questions
Exam 43: Animal Nervous Systems100 Questions
Exam 44: Animal Sensory Systems50 Questions
Exam 45: Animal Movement40 Questions
Exam 46: Chemical Signals in Animals59 Questions
Exam 47: Animal Reproduction and Development104 Questions
Exam 48: The Immune System in Animals77 Questions
Exam 49: An Introduction to Ecology40 Questions
Exam 50: Behavioral Ecology40 Questions
Exam 51: Population Ecology57 Questions
Exam 52: Community Ecology55 Questions
Exam 54: Biodiversity and Conservation Biology43 Questions
Select questions type
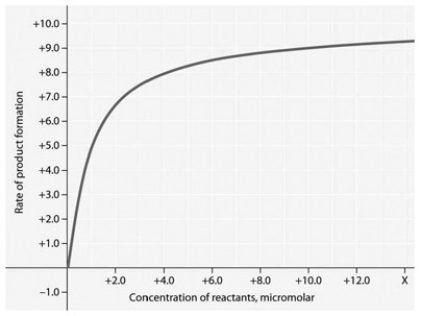 Rate of an enzyme-catalyzed reaction as a function of varying reactant
Concentration, with the concentration of enzyme constant.
In the accompanying figure, why does the reaction rate plateau at higher reactant concentrations?
Rate of an enzyme-catalyzed reaction as a function of varying reactant
Concentration, with the concentration of enzyme constant.
In the accompanying figure, why does the reaction rate plateau at higher reactant concentrations?
(Multiple Choice)
4.9/5  (42)
(42)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure. 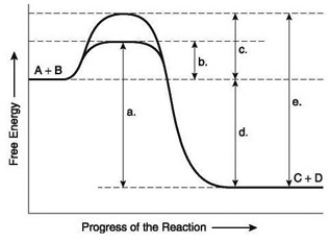 -You have isolated a previously unstudied protein, identified its complete structure in detail, and determined that it catalyzes the breakdown of a large substrate. You notice it has two binding sites. One of these is large, apparently the bonding site for the large substrate; the other is small, possibly a binding site for a regulatory molecule. What do these findings tell you about the mechanism of this protein?
-You have isolated a previously unstudied protein, identified its complete structure in detail, and determined that it catalyzes the breakdown of a large substrate. You notice it has two binding sites. One of these is large, apparently the bonding site for the large substrate; the other is small, possibly a binding site for a regulatory molecule. What do these findings tell you about the mechanism of this protein?
(Multiple Choice)
4.8/5  (35)
(35)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -Which of the following in the figure would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
-Which of the following in the figure would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is TRUE for all exergonic reactions?
(Multiple Choice)
4.7/5  (44)
(44)
Use the following information to answer the question(s) below.
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
-Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?
(Multiple Choice)
4.9/5  (37)
(37)
When chemical, transport, or mechanical work is done by an organism, what happens to the heat generated?
(Multiple Choice)
4.7/5  (33)
(33)
The mathematical expression for the change in free energy of a system is ΔG =ΔH − TΔS. Which of the following is (are) correct?
(Multiple Choice)
4.8/5  (35)
(35)
When 10,000 molecules of ATP are hydrolyzed to ADP and i in a test tube, about half as much heat is liberated as when a cell hydrolyzes the same amount of ATP. Which of the following is the best explanation for this observation?
(Multiple Choice)
4.9/5  (37)
(37)
Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
(Multiple Choice)
4.9/5  (34)
(34)
An important group of peripheral membrane proteins are enzymes such as the phospholipases that cleave the head groups of phospholipids. What properties must these enzymes exhibit?
(Multiple Choice)
4.8/5  (40)
(40)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -Which of the following represents the difference between the free-energy content of the reactants and the free-energy content of the products in the figure?
-Which of the following represents the difference between the free-energy content of the reactants and the free-energy content of the products in the figure?
(Multiple Choice)
4.9/5  (34)
(34)
Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as ________.
(Multiple Choice)
4.7/5  (29)
(29)
Use the following information to answer the question(s) below.
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
-What is substance X?
(Multiple Choice)
4.9/5  (43)
(43)
Whenever energy is transformed, there is always an increase in the ________.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following statements is TRUE about enzyme-catalyzed reactions?
(Multiple Choice)
4.7/5  (35)
(35)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -Which of the following represents the activation energy needed for the noncatalyzed reverse reaction, C + D → A + B, in the figure?
-Which of the following represents the activation energy needed for the noncatalyzed reverse reaction, C + D → A + B, in the figure?
(Multiple Choice)
4.9/5  (25)
(25)
A solution of starch at room temperature does NOT readily decompose to form a solution of simple sugars because ________.
(Multiple Choice)
4.9/5  (42)
(42)
The following question(s) are based on the reaction A + B ↔ C + D shown in the accompanying figure.  -Which of the following represents the activation energy required for a noncatalyzed reaction in the figure?
-Which of the following represents the activation energy required for a noncatalyzed reaction in the figure?
(Multiple Choice)
4.8/5  (27)
(27)
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 60 of 60
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)