Exam 5: An Introduction to Carbohydrates
Exam 1: Biology and the Tree of Life37 Questions
Exam 2: Water and Carbon: the Chemical Basis of Life59 Questions
Exam 3: Protein Structure and Function59 Questions
Exam 4: Nucleic Acids and the Rna World43 Questions
Exam 5: An Introduction to Carbohydrates44 Questions
Exam 53: Ecosystems and Global Ecology57 Questions
Exam 6: Lipids, Membranes, and the First Cells59 Questions
Exam 7: Inside the Cell60 Questions
Exam 8: Energy and Enzymes: an Introduction to Metabolism60 Questions
Exam 9: Cellular Respiration and Fermentation61 Questions
Exam 10: Photosynthesis58 Questions
Exam 11: Cellcell Interactions52 Questions
Exam 12: The Cell Cycle59 Questions
Exam 13: Meiosis63 Questions
Exam 14: Mendel and the Gene60 Questions
Exam 15: Dna and the Gene: Synthesis and Repair51 Questions
Exam 16: How Genes Work48 Questions
Exam 17: Transcription, Rna Processing, and Translation58 Questions
Exam 18: Control of Gene Expression in Bacteria29 Questions
Exam 19: Control of Gene Expression in Eukaryotes56 Questions
Exam 20: The Molecular Revolution: Biotechnology and Beyond70 Questions
Exam 21: Genes, Development, and Evolution38 Questions
Exam 22: Evolution by Natural Selection38 Questions
Exam 23: Evolutionary Processes37 Questions
Exam 24: Speciation56 Questions
Exam 25: Phylogenies and the History of Life63 Questions
Exam 26: Bacteria and Archaea38 Questions
Exam 27: Protists37 Questions
Exam 28: Green Algae and Land Plants59 Questions
Exam 29: Fungi47 Questions
Exam 30: An Introduction to Animals48 Questions
Exam 31: Protostome Animals54 Questions
Exam 32: Deuterostome Animals60 Questions
Exam 33: Viruses44 Questions
Exam 34: Plant Form and Function46 Questions
Exam 35: Water and Sugar Transport in Plants47 Questions
Exam 36: Plant Nutrition54 Questions
Exam 37: Plant Sensory Systems, Signals, and Responses48 Questions
Exam 38: Plant Reproduction and Development51 Questions
Exam 39: Animal Form and Function53 Questions
Exam 40: Water and Electrolyte Balance in Animals60 Questions
Exam 41: Animal Nutrition94 Questions
Exam 42: Gas Exchange and Circulation93 Questions
Exam 43: Animal Nervous Systems100 Questions
Exam 44: Animal Sensory Systems50 Questions
Exam 45: Animal Movement40 Questions
Exam 46: Chemical Signals in Animals59 Questions
Exam 47: Animal Reproduction and Development104 Questions
Exam 48: The Immune System in Animals77 Questions
Exam 49: An Introduction to Ecology40 Questions
Exam 50: Behavioral Ecology40 Questions
Exam 51: Population Ecology57 Questions
Exam 52: Community Ecology55 Questions
Exam 54: Biodiversity and Conservation Biology43 Questions
Select questions type
Glucose (C6H12O6) has a single carbonyl group (-C=O) in its linear form. Based on the number of oxygen atoms in glucose, how many hydroxyl groups (-OH) would you expect glucose to have?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
B
Use the following paragraph to answer the corresponding question.
Masatomo Kawakubo et al. reported in Science in August 2004 that the human stomach contains a natural, carbohydrate-based antibiotic that probably protects a large portion of the population from various diseases caused by the bacterium Helicobacter pylori. This bacterium has been linked to peptic ulcers, gastritis, and stomach cancer. This naturally occurring antibiotic is described by Kawakubo as having a terminal α-1,4-linked N-acetylglucosamine (NAG), and it acts by inhibiting the biosynthesis of a major component of the cell wall in H. pylori. [SOURCE: M. Kawakubo et al., Science 305 (2004): 1003.]
Refer to the paragraph about Kawakubo's group. Kawakubo's group created a glycoprotein with a terminal NAG (i.e., a protein with NAG attached to its end). Their hypothesis is that the terminal NAG, and not the protein component, is responsible for the damage to the cell wall in H. pylori. What would be the most appropriate control for testing this hypothesis?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
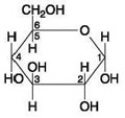 If two molecules of the general type shown in the accompanying figure were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be ________.
If two molecules of the general type shown in the accompanying figure were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be ________.
(Multiple Choice)
4.9/5  (32)
(32)
You isolate an organic molecule that contains C, H, O, N, and S. This molecule ________.
(Multiple Choice)
4.9/5  (38)
(38)
Which polysaccharide is an important component in the structure of many animals and fungi?
(Multiple Choice)
4.8/5  (29)
(29)
Cell walls are used by many different organisms for protection from their environment and structural support. These cell walls must obviously be insoluble in water; otherwise, they would dissolve the first time an organism got wet. Which of the following carbohydrates would you expect to be most soluble in water?
(Multiple Choice)
4.8/5  (43)
(43)
A molecule with the chemical formula C6H12O6 is probably a ________.
(Multiple Choice)
4.8/5  (30)
(30)
A glycosidic linkage is analogous to which of the following in proteins?
(Multiple Choice)
4.8/5  (23)
(23)
Which of the following best explains why "carbs" (carbohydrates) are advertised by manufacturers of candy bars and sports drinks as a "quick energy boost"?
(Multiple Choice)
4.8/5  (34)
(34)
Bacteria, insects, and plants use carbohydrates to build structures. Which of the following is TRUE of structural carbohydrates?
(Multiple Choice)
4.8/5  (24)
(24)
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a molecule made by linking three glucose molecules together by dehydration reactions?
(Multiple Choice)
4.8/5  (36)
(36)
If you were going to develop a new antibiotic against bacteria, you would probably need to become an expert on which of these carbohydrates?
(Multiple Choice)
4.9/5  (31)
(31)
A primary function of carbohydrates attached to the glycoproteins and glycolipids of animal cell membranes is to ________.
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following would you expect to have the most free energy per gram? The most free energy per gram would be found in a molecule with ________.
(Multiple Choice)
4.9/5  (34)
(34)
Enzymes that readily break starch apart cannot hydrolyze the glycosidic linkages found in cellulose. Why is this logical?
(Multiple Choice)
4.9/5  (34)
(34)
Which of these best reflects the following relationship: monosaccharide versus polysaccharide?
(Multiple Choice)
4.9/5  (37)
(37)
Lactose, a sugar in milk, is composed of one glucose molecule joined by a glycosidic linkage to one galactose molecule. How is lactose classified?
(Multiple Choice)
4.7/5  (39)
(39)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)