Exam 3: Water and Life
Exam 1: Introduction: Themes in the Study of Life64 Questions
Exam 2: The Chemical Context of Life83 Questions
Exam 3: Water and Life70 Questions
Exam 4: Carbon and the Molecular Diversity of Life71 Questions
Exam 5: The Structure and Function of Large Biological Molecules109 Questions
Exam 6: A Tour of the Cell80 Questions
Exam 7: Membrane Structure and Function80 Questions
Exam 8: An Introduction to Metabolism80 Questions
Exam 9: Cellular Respiration and Fermentation107 Questions
Exam 10: Photosynthesis81 Questions
Exam 11: Cell Communication69 Questions
Exam 12: The Cell Cycle79 Questions
Exam 13: Meiosis and Sexual Life Cycles70 Questions
Exam 14: Mendel and the Gene Idea73 Questions
Exam 15: The Chromosomal Basis of Inheritance61 Questions
Exam 16: The Molecular Basis of Inheritance57 Questions
Exam 17: From Gene to Protein83 Questions
Exam 18: Regulation of Gene Expression99 Questions
Exam 19: Viruses47 Questions
Exam 20: Biotechnology72 Questions
Exam 21: Genomes and Their Evolution42 Questions
Exam 22: Descent with Modification: A Darwinian View of Life55 Questions
Exam 23: The Evolution of Populations78 Questions
Exam 24: The Origin of Species63 Questions
Exam 25: The History of Life on Earth75 Questions
Exam 26: Phylogeny and the Tree of Life73 Questions
Exam 27: Bacteria and Archaea78 Questions
Exam 28: Protists76 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land74 Questions
Exam 30: Plant Diversity II: The Evolution of Seed Plants102 Questions
Exam 31: Fungi89 Questions
Exam 32: An Overview of Animal Diversity74 Questions
Exam 33: An Introduction to Invertebrates93 Questions
Exam 34: The Origin and Evolution of Vertebrates109 Questions
Exam 35: Plant Structure, Growth, and Development67 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants82 Questions
Exam 37: Soil and Plant Nutrition83 Questions
Exam 38: Angiosperm Reproduction and Biotechnology86 Questions
Exam 39: Plant Responses to Internal and External Signals108 Questions
Exam 40: Basic Principles of Animal Form and Function77 Questions
Exam 41: Animal Nutrition64 Questions
Exam 42: Circulation and Gas Exchange90 Questions
Exam 43: The Immune System100 Questions
Exam 44: Osmoregulation and Excretion69 Questions
Exam 45: Hormones and the Endocrine System72 Questions
Exam 46: Animal Reproduction94 Questions
Exam 47: Animal Development92 Questions
Exam 48: Neurons, Synapses, and Signaling73 Questions
Exam 49: Nervous Systems65 Questions
Exam 50: Sensory and Motor Mechanisms82 Questions
Exam 51: Animal Behavior69 Questions
Exam 52: An Introduction to Ecology and the Biosphere73 Questions
Exam 53: Population Ecology79 Questions
Exam 54: Community Ecology77 Questions
Exam 55: Ecosystems and Restoration Ecology81 Questions
Exam 56: Conservation Biology and Global Change67 Questions
Select questions type
Which type of bond must be broken for water to vaporize?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
How many grams of acetic acid (C₂H₄O₂)would you use to make 10 L of a 0.1 M aqueous solution of acetic acid? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
D
Increased atmospheric CO₂ concentrations might have what effect on seawater?
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
D
One idea to mitigate the effects of burning fossil fuels on atmospheric CO₂ concentrations is to pipe liquid CO₂ into the ocean at depths of 2,500 feet or greater. At the high pressures at such depths, CO₂ is heavier than water. What potential effects might result from implementing such a scheme?
(Multiple Choice)
4.9/5  (38)
(38)
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)
(Multiple Choice)
4.7/5  (36)
(36)
How would acidification of seawater affect marine organisms?
(Multiple Choice)
4.7/5  (36)
(36)
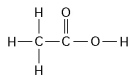 -How many grams would be equal to 1 mol of the compound shown in the figure above?
(carbon = 12, oxygen = 16, hydrogen = 1)
-How many grams would be equal to 1 mol of the compound shown in the figure above?
(carbon = 12, oxygen = 16, hydrogen = 1)
(Multiple Choice)
4.8/5  (26)
(26)
Research indicates that acid precipitation can damage living organisms by
(Multiple Choice)
4.8/5  (30)
(30)
What is the hydrogen ion [H⁺] concentration of a solution of pH 8?
(Multiple Choice)
5.0/5  (30)
(30)
Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol), so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
(Multiple Choice)
4.9/5  (38)
(38)
The slight negative charge at one end of one water molecule is attracted to the slight positive charge of another water molecule. What is this attraction called?
(Multiple Choice)
4.8/5  (34)
(34)
Consider two solutions: solution X has a pH of 4; solution Y has a pH of 7. From this information, we can reasonably conclude that
(Multiple Choice)
4.8/5  (37)
(37)
Sulfur is in the same column of the periodic table as oxygen, but has electronegativity similar to carbon. Compared to water molecules, molecules of H₂S
(Multiple Choice)
4.8/5  (35)
(35)
Temperature usually increases when water condenses. Which behavior of water is most directly responsible for this phenomenon?
(Multiple Choice)
4.9/5  (42)
(42)
You have two beakers. One contains pure water, the other contains pure methanol (wood alcohol). The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. You pour crystals of table salt (NaCl)into each beaker. Predict what will happen.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements is true about buffer solutions?
(Multiple Choice)
4.7/5  (38)
(38)
 -How many grams of the compound in the figure above would be required to make 2.5 L of a 1 M solution?
(carbon = 12, oxygen = 16, hydrogen = 1)
-How many grams of the compound in the figure above would be required to make 2.5 L of a 1 M solution?
(carbon = 12, oxygen = 16, hydrogen = 1)
(Multiple Choice)
4.8/5  (29)
(29)
You have a freshly prepared 0.1 M solution of glucose in water. Each liter of this solution contains how many glucose molecules?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)