Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Draw a plausible curved-arrow mechanism to show the base-catalyzed hydrolysis of the phosphate triester, then draw the hydrolysis products.
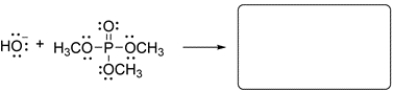
(Essay)
4.9/5  (33)
(33)
Predict the major organic product(s) for the reaction. If the reaction will not occur, write NR and explain why.
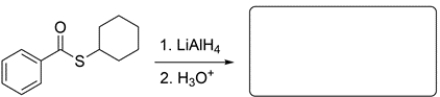
(Essay)
4.8/5  (39)
(39)
Glutathione is a coenzyme consisting of the tripeptide Glu-Cys-Gly. The first step of the synthesis of glutathione is catalyzed by glutamyl cysteine synthetase, where glutamate reacts with ATP to form an acyl phosphate. In the second step, cysteine reacts with the acyl phosphate to form -Glu-Cys and phosphate ion. Note, the -Glu indicates the reaction occurs on the sidechain.
Draw the acyl phosphate formed in the first step, and then draw a plausible curved-arrow mechanism showing how the acyl phosphate can be converted to -Glu-Cys.

(Essay)
4.8/5  (35)
(35)
Calculate the ΔG°´ for the phosphorylation of fructose-6-phosphate to fructose 1,6-biphosphate using ATP, given the following.

(Essay)
4.9/5  (24)
(24)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)