Exam 8: Nomenclature and Noncovalent Intermolecular Interactions
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Which structure has the lowest melting point of the various 18-carbon fatty acids given?
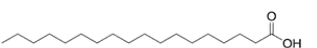 (A) stearic acid (found in lard)
(A) stearic acid (found in lard)
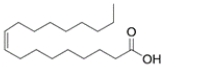 (B) oleic acid (found in olive oil)
(B) oleic acid (found in olive oil)
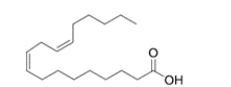 (C) linoleic acid (found in grapeseed oil)
(C) linoleic acid (found in grapeseed oil)
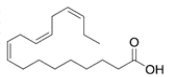 (D) -linoleic acid (found in flaxseed oil)
(D) -linoleic acid (found in flaxseed oil)
(Multiple Choice)
4.8/5  (38)
(38)
Compounds consisting of molecules that can both donate and accept hydrogen bonds have higher boiling points than isomeric compounds that cannot donate hydrogen bonds because
(Multiple Choice)
4.9/5  (34)
(34)
Until the FDA rescinded the permit for its use in 2002, perflubron, a mixture of 1-bromoperfluorooctyl bromide [CF3(CF2)6CF2Br] and 1-bromoperfluoroodecyl bromide [CF3(CF2)8CF2Br], was being investigated for possible use as artificial blood during surgery. Perflubron dissolves substantial amounts of oxygen; a mouse can breathe when submerged in oxygenated perflubron. Perflubron is completely insoluble in water. To make it compatible with the aqueous biological environment, it had to be suspended in water in such a way that it would form an emulsion and not separate into layers. (In an emulsion, water-insoluble substances are suspended as small particles that do not form a separate layer.) Addition of lecithin, a phospholipid from egg yolks, met this objective.
![Until the FDA rescinded the permit for its use in 2002, perflubron, a mixture of 1-bromoperfluorooctyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>6</sub>CF<sub>2</sub>Br] and 1-bromoperfluoroodecyl bromide [CF<sub>3</sub>(CF<sub>2</sub>)<sub>8</sub>CF<sub>2</sub>Br], was being investigated for possible use as artificial blood during surgery. Perflubron dissolves substantial amounts of oxygen; a mouse can breathe when submerged in oxygenated perflubron. Perflubron is completely insoluble in water. To make it compatible with the aqueous biological environment, it had to be suspended in water in such a way that it would form an emulsion and not separate into layers. (In an emulsion, water-insoluble substances are suspended as small particles that do not form a separate layer.) Addition of lecithin, a phospholipid from egg yolks, met this objective. Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.](https://storage.examlex.com/TBMC1048/11edaded_7d16_96fe_a31a_03338cb64c69_TBMC1048_00.jpg) Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
Explain the action of lecithin. As part of your explanation, use a diagram to propose a general structure of a perflubron-containing particle.
(Essay)
4.8/5  (24)
(24)
Name the compound. Include the appropriate stereochemical designation in the name.
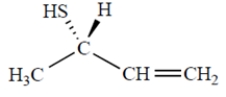
(Short Answer)
5.0/5  (39)
(39)
Given four compounds with the same branching pattern and similar molecular masses, which compound has the highest boiling point?
(A)
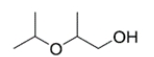 (B)
(B)
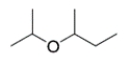 (C)
(C)
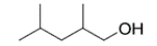 (D)
(D)
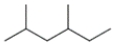
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)