Exam 7: Cyclic Compounds and Reaction Stereochemistry
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Cis-1,3-dimethylcyclohexane contains considerably smaller percentage of diaxial conformation than trans-1,2-dimethylcyclohexane. However, cis-cyclohexane-1,3-diol has a higher percentage diaxial conformation than trans-cyclohexane-1,2-diol. (For purposes of this problem, you can assume that an -OH group and a -CH3 group have about the same size.) Explain this difference. A drawing of the diaxial chair conformation of cis-cyclohexane-1,3-diol must be part of your answer.
(Essay)
4.8/5  (34)
(34)
Consider bromine addition to alkene A below, which is the pure enantiomer. As a pure enantiomer, it is optically active, and measurement of its optical activity shows that it has (+) optical rotation.
(Essay)
4.8/5  (38)
(38)
Consider the following reaction and its stereochemistry.
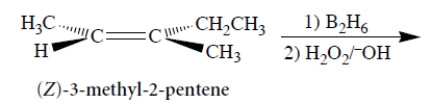 Select the correct statements.
Select the correct statements.
(Multiple Choice)
4.8/5  (41)
(41)
Complete the following reaction by giving the structures of the missing organic products.
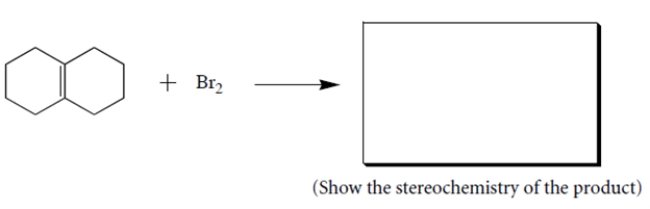
(Essay)
4.8/5  (31)
(31)
Draw the two chair conformations of cyclohexanol and circle the more stable conformation of the two. You only need to show the carbon-hydrogen bond at the carbon bearing the OH group (†). Bonds must be properly positioned for credit.
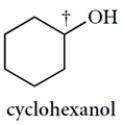
(Essay)
4.7/5  (33)
(33)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)