Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Consider this Brønsted acid-base equilibrium:
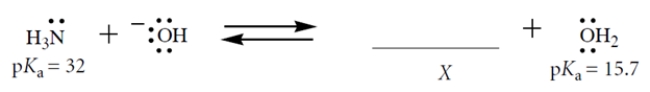 1. Fill in the structure of X; show formal charge and all valence electrons.
2. What is Keq for this reaction for the left-to-right direction?
3. If the reaction starts out with 1 M NaOH and 0.5 M NH3 in the solvent water (55.5 M), which species (other than water) is present in the highest concentration at equilibrium?
1. Fill in the structure of X; show formal charge and all valence electrons.
2. What is Keq for this reaction for the left-to-right direction?
3. If the reaction starts out with 1 M NaOH and 0.5 M NH3 in the solvent water (55.5 M), which species (other than water) is present in the highest concentration at equilibrium?
(Essay)
5.0/5  (26)
(26)
Equal amount of compounds A and B (0.01 mol each) are dissolved together in 1 L of water, and this equilibrium is established:
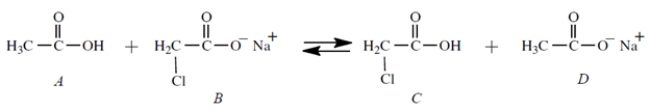 What are the major species in solution at equilibrium? (Select all that apply)
What are the major species in solution at equilibrium? (Select all that apply)
(Multiple Choice)
4.9/5  (30)
(30)
Determine the standard free-energy change of a reaction with an equilibrium constant of 250, given that 2.3RT at 298 K is 5.7 kJ mol-1. ΔG° = ________________ kJ mol-1.
(Short Answer)
5.0/5  (34)
(34)
Predict the approximate equilibrium constant (as a power of 10) for the reaction.
(Short Answer)
4.8/5  (30)
(30)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)