Exam 8: An Introduction to Metabolism
Exam 1: Introduction: Themes in the Study of Life64 Questions
Exam 2: The Chemical Context of Life83 Questions
Exam 3: Water and Life70 Questions
Exam 4: Carbon and the Molecular Diversity of Life71 Questions
Exam 5: The Structure and Function of Large Biological Molecules109 Questions
Exam 6: A Tour of the Cell80 Questions
Exam 7: Membrane Structure and Function80 Questions
Exam 8: An Introduction to Metabolism80 Questions
Exam 9: Cellular Respiration and Fermentation107 Questions
Exam 10: Photosynthesis81 Questions
Exam 11: Cell Communication69 Questions
Exam 12: The Cell Cycle79 Questions
Exam 13: Meiosis and Sexual Life Cycles70 Questions
Exam 14: Mendel and the Gene Idea73 Questions
Exam 15: The Chromosomal Basis of Inheritance61 Questions
Exam 16: The Molecular Basis of Inheritance57 Questions
Exam 17: From Gene to Protein83 Questions
Exam 18: Regulation of Gene Expression99 Questions
Exam 19: Viruses47 Questions
Exam 20: Biotechnology72 Questions
Exam 21: Genomes and Their Evolution42 Questions
Exam 22: Descent with Modification: A Darwinian View of Life55 Questions
Exam 23: The Evolution of Populations78 Questions
Exam 24: The Origin of Species63 Questions
Exam 25: The History of Life on Earth75 Questions
Exam 26: Phylogeny and the Tree of Life73 Questions
Exam 27: Bacteria and Archaea78 Questions
Exam 28: Protists76 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land74 Questions
Exam 30: Plant Diversity II: The Evolution of Seed Plants102 Questions
Exam 31: Fungi89 Questions
Exam 32: An Overview of Animal Diversity74 Questions
Exam 33: An Introduction to Invertebrates93 Questions
Exam 34: The Origin and Evolution of Vertebrates109 Questions
Exam 35: Plant Structure, Growth, and Development67 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants82 Questions
Exam 37: Soil and Plant Nutrition83 Questions
Exam 38: Angiosperm Reproduction and Biotechnology86 Questions
Exam 39: Plant Responses to Internal and External Signals108 Questions
Exam 40: Basic Principles of Animal Form and Function77 Questions
Exam 41: Animal Nutrition64 Questions
Exam 42: Circulation and Gas Exchange90 Questions
Exam 43: The Immune System100 Questions
Exam 44: Osmoregulation and Excretion69 Questions
Exam 45: Hormones and the Endocrine System72 Questions
Exam 46: Animal Reproduction94 Questions
Exam 47: Animal Development92 Questions
Exam 48: Neurons, Synapses, and Signaling73 Questions
Exam 49: Nervous Systems65 Questions
Exam 50: Sensory and Motor Mechanisms82 Questions
Exam 51: Animal Behavior69 Questions
Exam 52: An Introduction to Ecology and the Biosphere73 Questions
Exam 53: Population Ecology79 Questions
Exam 54: Community Ecology77 Questions
Exam 55: Ecosystems and Restoration Ecology81 Questions
Exam 56: Conservation Biology and Global Change67 Questions
Select questions type
Which of the following is true of metabolism in its entirety in all organisms?
(Multiple Choice)
4.8/5  (40)
(40)
Biological evolution of life on Earth, from simple prokaryote-like cells to large, multicellar eukaryotic organisms,
(Multiple Choice)
4.9/5  (33)
(33)
The following questions are from the end-of-chapter "Test Your Understanding" section in Chapter 8 of the textbook.
-Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as ________ is to ________.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following statements is true about enzyme-catalyzed reactions?
(Multiple Choice)
4.8/5  (32)
(32)
What is the difference (if any)between the structure of ATP and the structure of the precursor of the A nucleotide in RNA?
(Multiple Choice)
4.7/5  (31)
(31)
The following questions are from the end-of-chapter "Test Your Understanding" section in Chapter 8 of the textbook.
-Some bacteria are metabolically active in hot springs because
(Multiple Choice)
4.9/5  (37)
(37)
A chemical reaction that has a positive ΔG is correctly described as
(Multiple Choice)
4.9/5  (40)
(40)
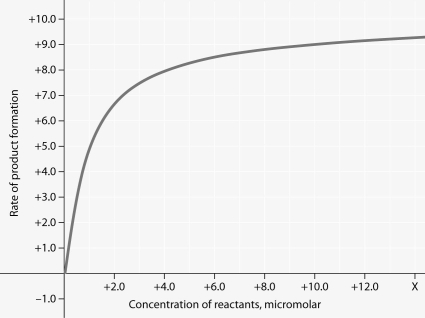 Rate of an enzyme-catalyzed reaction as a function of varying reactant
concentration, with the concentration of enzyme constant.
-For the enzyme-catalyzed reaction shown in the figure, which of these treatments will cause the greatest increase in the rate of the reaction, if the initial reactant concentration is 1.0 micromolar?
Rate of an enzyme-catalyzed reaction as a function of varying reactant
concentration, with the concentration of enzyme constant.
-For the enzyme-catalyzed reaction shown in the figure, which of these treatments will cause the greatest increase in the rate of the reaction, if the initial reactant concentration is 1.0 micromolar?
(Multiple Choice)
4.8/5  (32)
(32)
For living organisms, which of the following is an important consequence of the first law of thermodynamics?
(Multiple Choice)
4.8/5  (38)
(38)
Mutations that result in single amino acid substitutions in an enzyme
(Multiple Choice)
4.9/5  (38)
(38)
Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as a(n)
(Multiple Choice)
4.9/5  (45)
(45)
How does a noncompetitive inhibitor decrease the rate of an enzyme reaction?
(Multiple Choice)
4.9/5  (36)
(36)
A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases although they don't often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10⁻⁷ while the concentration in the SR is 10⁻², then how is the ATPase acting?
(Multiple Choice)
4.7/5  (32)
(32)
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following?
(Multiple Choice)
4.8/5  (25)
(25)
Which of the following statements regarding enzymes is true?
(Multiple Choice)
4.8/5  (31)
(31)
Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?
(Multiple Choice)
4.9/5  (30)
(30)
For the hydrolysis of ATP to ADP + 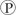 ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products). In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and
ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products). In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and 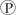 ᵢ under cellular conditions?
ᵢ under cellular conditions?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 41 - 60 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)