Exam 13: Introduction to Spectroscopy
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
When the epoxy thiol is treated with dilute acid in ethanol solution, an isomer is formed that shows a broad stretch at 3400 cm-1 in its IR spectrum. Using mechanistic reasoning with the curved-arrow notation, predict the structure of the product and its stereochemistry.
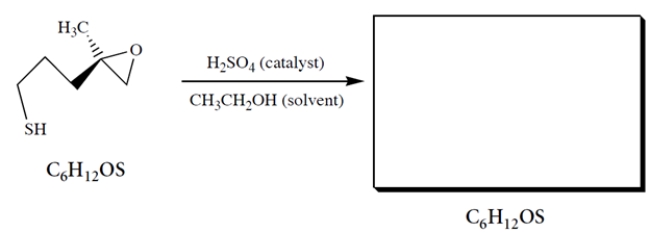
(Essay)
4.8/5  (33)
(33)
The base peak of 2,2-dimethylpentane is at m/z 57, which corresponds to a composition of C4H9.
(a) Suggest a structure for the fragment that accounts for this peak.
(b) Offer a reason that this fragment is so abundant.
(c) Give a mechanism that shows the formation of this fragment.
(Essay)
4.8/5  (32)
(32)
Menthol is obtained from oils of peppermint and spearmint and is often added to cough drops to give a minty, cooling sensation. In the electron ionization mass spectrum of menthol, the molecular ion peak is not observed, but the largest fragment has an m/z 138 at 27% relative abundance and an ion at m/z 139 with a 3% relative abundance. Given the two possible compounds, identify which is menthol and explain how you arrived at that conclusion.
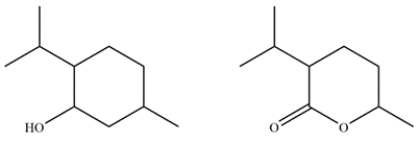
(Essay)
4.8/5  (26)
(26)
Which of the compounds would have the strongest C=C stretching absorption in its IR spectrum?
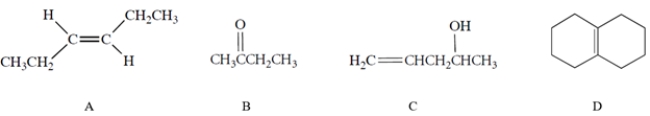
(Multiple Choice)
4.9/5  (38)
(38)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)