Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Outline the synthesis of dicyclopentyl ether using alkoxymercuration-reduction.
(Essay)
4.7/5  (29)
(29)
Outline a multistep synthesis of the given alkene from acetylene and any other reagents.
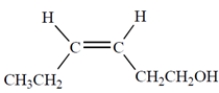 You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.
You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.
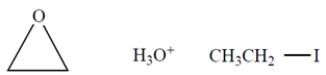
(Essay)
4.7/5  (39)
(39)
Complete the diagram by supplying structures for all the missing compounds.
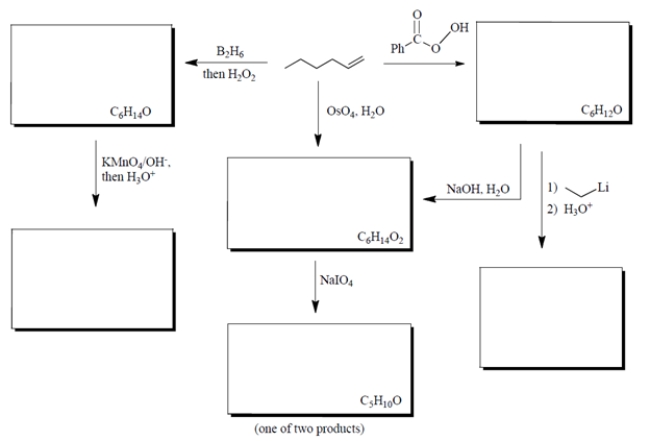
(Essay)
4.8/5  (37)
(37)
a. Only one of the two diastereomers (formula C12H23OBr) will react with NaOH to form a product X with the formula C12H22O. Identify the reactive diastereomer by circling it and labeling it with the letter A. Draw the product X in its chair conformation.
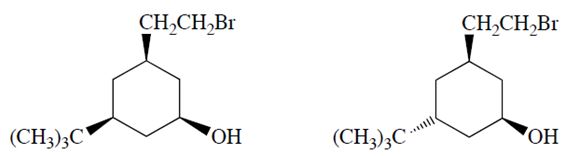 b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.
b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.
(Essay)
4.8/5  (37)
(37)
Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found in cigarette smoke that is converted by enzymes in the body to a diol epoxide.
![Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found in cigarette smoke that is converted by enzymes in the body to a diol epoxide. This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H<sub>2</sub>O is the acid where acids and bases are necessary.](https://storage.examlex.com/TBMC1048/11edaded_7d3b_35c7_a31a_79fa30fdaebc_TBMC1048_00.jpg) This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
(Essay)
4.9/5  (34)
(34)
Showing 21 - 25 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)