Exam 1: Chemical Bonding and Chemical Structure
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Give the formal charge on the phosphorus atom in this structure. (Phosphorus is in group 5A directly under nitrogen in the periodic table.)
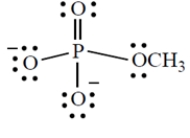
(Short Answer)
4.9/5  (31)
(31)
Assume that the structure of BF3 (boron trifluoride, or trifluoroborane) is correctly predicted by the VSEPR rules.
1. What is the predicted structure? (Show all unpaired valence electrons.)
2. The B-F bond is exceptionally strong. This suggests that it has some double-bond character. Draw resonance structures for BF3 that show that the three B-F bonds share some double-bond character. Be sure to show formal charges and all unshared electrons.
3. What is the polarity of the B-F bond? (In which direction is the bond dipole?) How do you know?
(Essay)
4.8/5  (36)
(36)
Consider this compound:
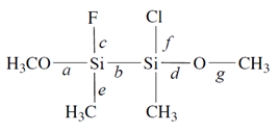 1. Of the labeled bonds, the longest bond is ________________.
2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.
1. Of the labeled bonds, the longest bond is ________________.
2. The most polar bond is ________________.
3. The bonding geometry at the silicon atoms is ________________.
4. The hybridization of the silicon atoms is ________________.
(Essay)
4.8/5  (39)
(39)
Consider this structure:
Which bond represents an sp-sp2 carbon-carbon bond?
(Short Answer)
4.9/5  (34)
(34)
Showing 21 - 26 of 26
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)