Exam 3: Protein Structure and Function
Exam 1: Biology and the Tree of Life35 Questions
Exam 2: Water and Carbon: the Chemical Basis of Life51 Questions
Exam 3: Protein Structure and Function54 Questions
Exam 4: Nucleic Acids and the Rna World40 Questions
Exam 5: An Introduction to Carbohydrates40 Questions
Exam 6: Lipids, membranes, and the First Cells54 Questions
Exam 7: Inside the Cell38 Questions
Exam 8: Cell-Cell Interactions38 Questions
Exam 9: Cellular Respiration and Fermentation38 Questions
Exam 10: Photosynthesis39 Questions
Exam 11: The Cell Cycle39 Questions
Exam 12: Meiosis39 Questions
Exam 13: Mendel and the Gene42 Questions
Exam 14: Dna and the Gene: Synthesis and Repair39 Questions
Exam 15: How Genes Work39 Questions
Exam 16: Transcription, RNA Processing, and Translation39 Questions
Exam 17: Control of Gene Expression in Bacteria38 Questions
Exam 18: Control of Gene Expression in Eukaryotes39 Questions
Exam 19: Analyzing and Engineering Genes41 Questions
Exam 20: Genomics41 Questions
Exam 21: Principles of Development39 Questions
Exam 22: An Introduction to Animal Development40 Questions
Exam 23: An Introduction to Plant Development37 Questions
Exam 24: Evolution by Natural Selection42 Questions
Exam 25: Evolutionary Processes50 Questions
Exam 26: Speciation41 Questions
Exam 27: Phylogenies and the History of Life43 Questions
Exam 28: Bacteria and Archaea38 Questions
Exam 29: Protists36 Questions
Exam 30: Green Algae and Land Plants54 Questions
Exam 31: Fungi40 Questions
Exam 32: An Introduction to Animals42 Questions
Exam 33: Protostome Animals38 Questions
Exam 34: Deuterostome Animals43 Questions
Exam 35: Viruses35 Questions
Exam 36: Plant Form and Function36 Questions
Exam 37: Water and Sugar Transport in Plants42 Questions
Exam 38: Plant Nutrition37 Questions
Exam 39: Plant Sensory Systems, signals, and Responses65 Questions
Exam 40: Plant Reproduction41 Questions
Exam 41: Animal Form and Function38 Questions
Exam 42: Water and Electrolyte Balance in Animals41 Questions
Exam 43: Animal Nutrition43 Questions
Exam 44: Gas Exchange and Circulation46 Questions
Exam 45: Electrical Signals in Animals40 Questions
Exam 46: Animal Sensory Systems and Movement43 Questions
Exam 47: Chemical Signals in Animals38 Questions
Exam 48: Animal Reproduction39 Questions
Exam 49: The Immune System in Animals38 Questions
Exam 50: An Introduction to Ecology41 Questions
Exam 51: Behavioural Ecology39 Questions
Exam 52: Population Ecology49 Questions
Exam 53: Community Ecology39 Questions
Exam 54: Ecosystems41 Questions
Exam 55: Biodiversity and Conservation Biology38 Questions
Select questions type
Consider the HIV enzyme called protease.The amino acid residues at the active site are highly hydrophobic.In designing a drug that would bind to the active site and jam it,researchers should use which type of molecule?
(Multiple Choice)
4.8/5  (40)
(40)
Figure 3.3
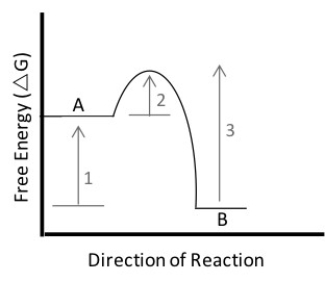 -In the figure above,which of the following statements is true?
-In the figure above,which of the following statements is true?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the experiment that Stanley Miller did to simulate chemical evolution.Recall that a glass flask held the reduced gases NH₃,CH₄,and H₂ and that the gases were exposed to electrical sparks.What is the null hypothesis in the experiment?
(Multiple Choice)
4.9/5  (37)
(37)
Suppose you discovered a new amino acid.Its R-group contains only hydrogen and carbon atoms.Predict the behavior of this amino acid.
(Multiple Choice)
4.7/5  (47)
(47)
Recent technological advances have made it more feasible than ever to work out the three-dimensional structure of proteins.There is intense interest in this research field,called structural biology.Why?
(Multiple Choice)
4.8/5  (42)
(42)
The aquaporin family of proteins plays a major role in the transport of water all over the body.During the folding process of these proteins,α-helices start forming as
(Multiple Choice)
4.9/5  (39)
(39)
An enzyme has a total of four active sites.When you denature the molecule and study its composition,you find that each active site occurs on a different polypeptide.Which of the following hypotheses does this observation support?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following best describes the first living entity-the one responsible for the origin of life?
(Multiple Choice)
4.7/5  (30)
(30)
What is the process component of the theory of chemical evolution?
(Multiple Choice)
4.7/5  (33)
(33)
You are studying a protein that is shaped like a doughnut.The shape is a function of which level(s)of protein structure?
(Multiple Choice)
4.8/5  (35)
(35)
What is the pattern component of the theory of chemical evolution?
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following is not true when comparing an uncatalyzed reaction to the same reaction with a catalyst?
(Multiple Choice)
4.8/5  (33)
(33)
You are studying reaction X.Which of the following scenarios would likely NOT help you to increase the rate of this reaction?
(Multiple Choice)
4.9/5  (26)
(26)
Refer to the following paragraph and figure 3.1 to answer the following questions.
![Refer to the following paragraph and figure 3.1 to answer the following questions. Figure 3.1 Since structure correlates so well with function, biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it. One of the most powerful techniques in existence today is X-ray crystallography. The main difficulty with this technique is getting the protein to crystallize. Once crystallized, the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein. This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin, which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans. The structure (schematically shown above, where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane. Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment. [Figure adapted from K. Palczewski et al., Science 289 (2000): 739.] -How many times does the protein in Figure 3.1 cross the cell membrane?](https://storage.examlex.com/TB3733/11ea46a8_f568_afc5_95d4_292e393386ff_TB3733_00_TB3733_00_TB3733_00_TB3733_00_TB3733_00_TB3733_00.jpg) Figure 3.1
Since structure correlates so well with function, biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it. One of the most powerful techniques in existence today is X-ray crystallography. The main difficulty with this technique is getting the protein to crystallize. Once crystallized, the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein. This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin, which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans. The structure (schematically shown above, where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane. Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment. [Figure adapted from K. Palczewski et al., Science 289 (2000): 739.]
-How many times does the protein in Figure 3.1 cross the cell membrane?
Figure 3.1
Since structure correlates so well with function, biochemists are constantly looking for new ways to probe the complex structure of proteins in order to understand what they do and how they do it. One of the most powerful techniques in existence today is X-ray crystallography. The main difficulty with this technique is getting the protein to crystallize. Once crystallized, the protein is bombarded with X-rays to create a pattern that can be analyzed mathematically to determine the three-dimensional structure of the protein. This analysis has been performed by Krzysztof Palczewski on the protein rhodopsin, which is a light-sensitive protein found in species ranging from ancient bacteria (archaea)to humans. The structure (schematically shown above, where each letter represents an amino acid)is characterized by a single polypeptide chain with several α-helical segments that loop back and forth across the cell membrane. Another notable feature is the disulfide bond (-S-S-)that can be seen at the bottom of the third transmembrane segment. [Figure adapted from K. Palczewski et al., Science 289 (2000): 739.]
-How many times does the protein in Figure 3.1 cross the cell membrane?
(Multiple Choice)
5.0/5  (41)
(41)
Showing 21 - 40 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)